Microstructure and Gravimetric Analysis of Hydrogen Behaviour in Aerospace Alloys for Initial Research of Kerosene Alternatives
Abstract
Commercial aircraft engines produce a high amount of carbon in the air during the exhaustion of kerosene; however, a replacement of kerosene with hydrogen can greatly contribute to reducing carbon emissions in the atmosphere. This experiment is meaningful in presenting the possibility of using alloy made for commercial fuel delivery systems as hydrogen pipe alloy by direct H-exposure of Al6082-T6 and Hastelloy X at 18 bar with elevated temperatures. The experiment was carried out by comparing microstructural, crystallographic, and gravimetric changes after the H-exposure and the results demonstrated that Al6082-T6 can be used in lower-pressure hydrogen pipelines and Hastelloy X in higher- pressure and high-temperature pipelines in commercial fuel delivery systems.
Introduction
With the aviation industry striving to reduce carbon emissions, hydrogen has emerged as a promising alternative to kerosene for commercial aircraft. However, the interaction of hydrogen with aerospace alloys presents challenges, such as hydrogen embrittlement and cracking, which could compromise material integrity. This study explores the potential of two widely used aerospace alloys, Al6082-T6 and Hastelloy X, as materials for hydrogen pipelines under high pressure and elevated temperatures.
Background
Hydrogen embrittlement (HE) is a phenomenon that weakens metals due to hydrogen diffusion into their structure. The study aims to understand how hydrogen affects the microstructure and gravimetric properties of Al6082-T6 and Hastelloy X. By examining these alloys under high-pressure hydrogen conditions, this research provides insights into their suitability for hydrogen fuel delivery systems in aviation.
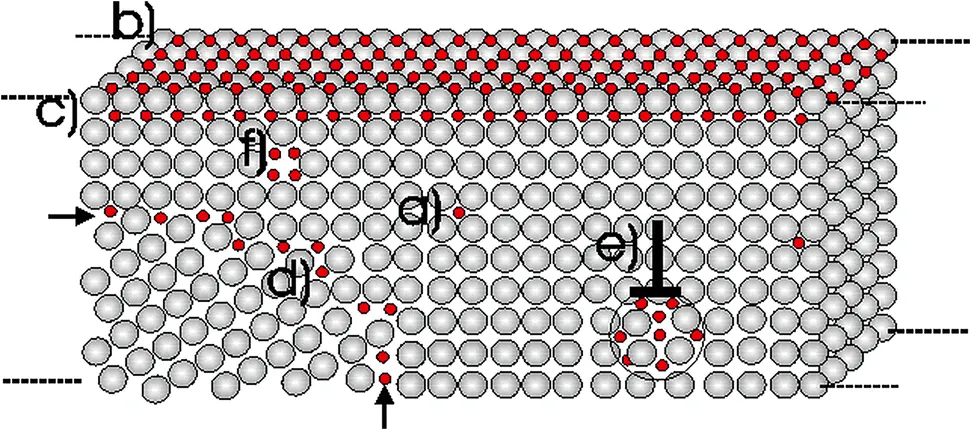
Figure 2: Hydrogen trapping site in steel: a) conventional solute hydrogen trapping site in matrix, b) hydrogen trapping site beneath the surface, c) subsurface, d) grain boundaries, e) edge dislocation and f) vacancies
Key Concepts:
•
Hydrogen Embrittlement: Mechanical degradation caused by hydrogen absorption.
•
Trapping Sites: Locations within metals where hydrogen is absorbed, such as grain boundaries and dislocations.
•
Precipitation Hardening: A process enhancing material strength through heat treatment.
Research Question
1.
How does hydrogen affect the microstructure, phase transformation, and mechanical properties of aerospace alloys (Al6082-T6 and Hastelloy X)?
2.
Can these alloys be effectively used for hydrogen pipelines in commercial aviation at high pressures and elevated temperatures?
3.
What are the gravimetric and crystallographic changes observed under hydrogen exposure conditions?
Aim
The study aims to evaluate the suitability of Al6082-T6 and Hastelloy X for hydrogen fuel delivery systems in aviation by investigating:
•
Hydrogen-induced microstructural and gravimetric changes.
•
The potential for hydrogen embrittlement or lattice distortion in these alloys.
•
The behaviour of hydrogen at high-pressure and high-temperature environments.
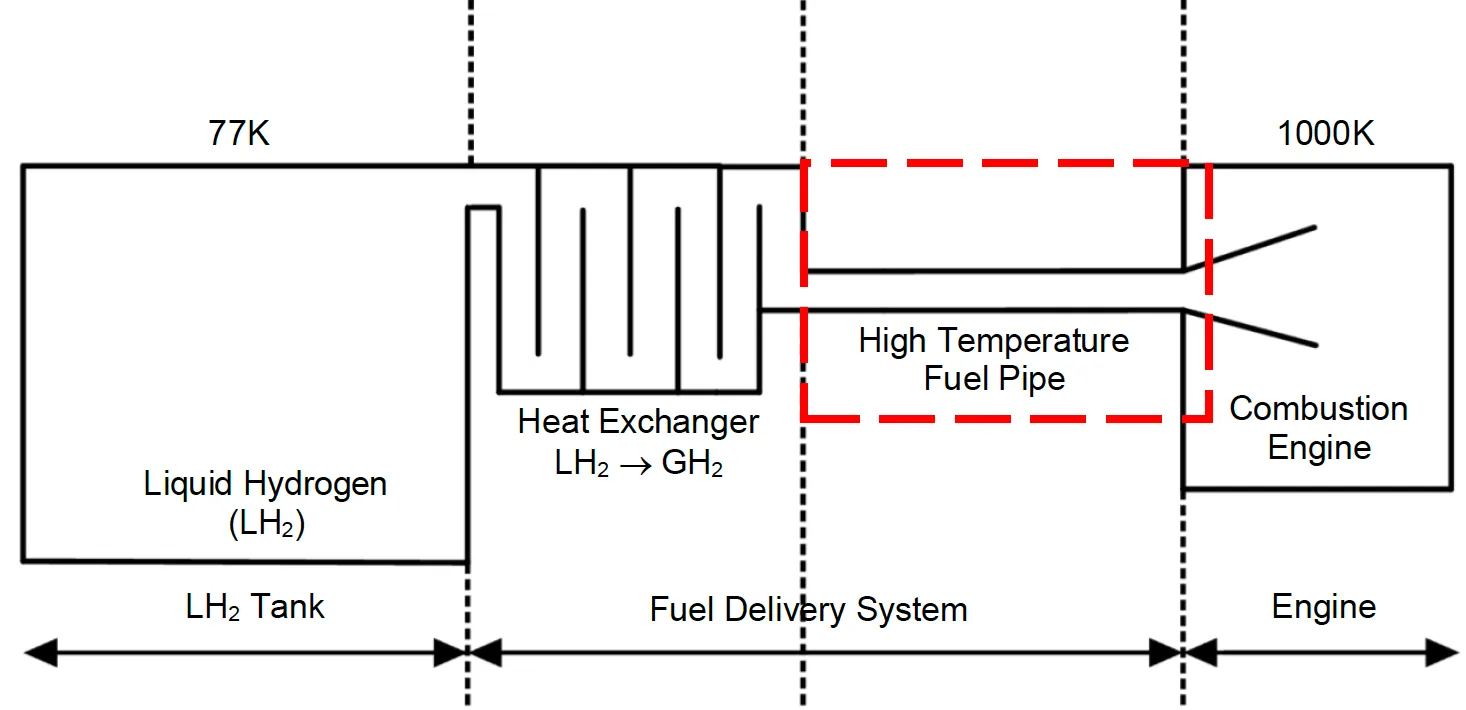
Figure 7: Schematic diagram of hydrogen combustion engine structure in this experiment: red box is the part of an engine that will be focused on this study
Significance
This research contributes to the understanding of hydrogen-metal interactions critical for aviation sustainability by:
•
Exploring safer and more efficient hydrogen delivery materials to replace kerosene pipelines.
•
Providing initial insights into alloy behaviour under extreme conditions, supporting future hydrogen-based aviation systems.
•
Encouraging material innovations that enable carbon-neutral energy solutions in aerospace applications.
Literature Review
Theoretical Framework and Logical Progression of the Study
Hydrogen Embrittlement (HE) is a critical phenomenon in evaluating the performance of metals under high-pressure hydrogen environments. It occurs as a result of hydrogen diffusion and trapping within the metal, leading to reduced structural stability. To understand this phenomenon, the study was designed based on the following theoretical concepts:
Key Theoretical Concepts
1.
Crystal Structure and Hydrogen Diffusion Pathways
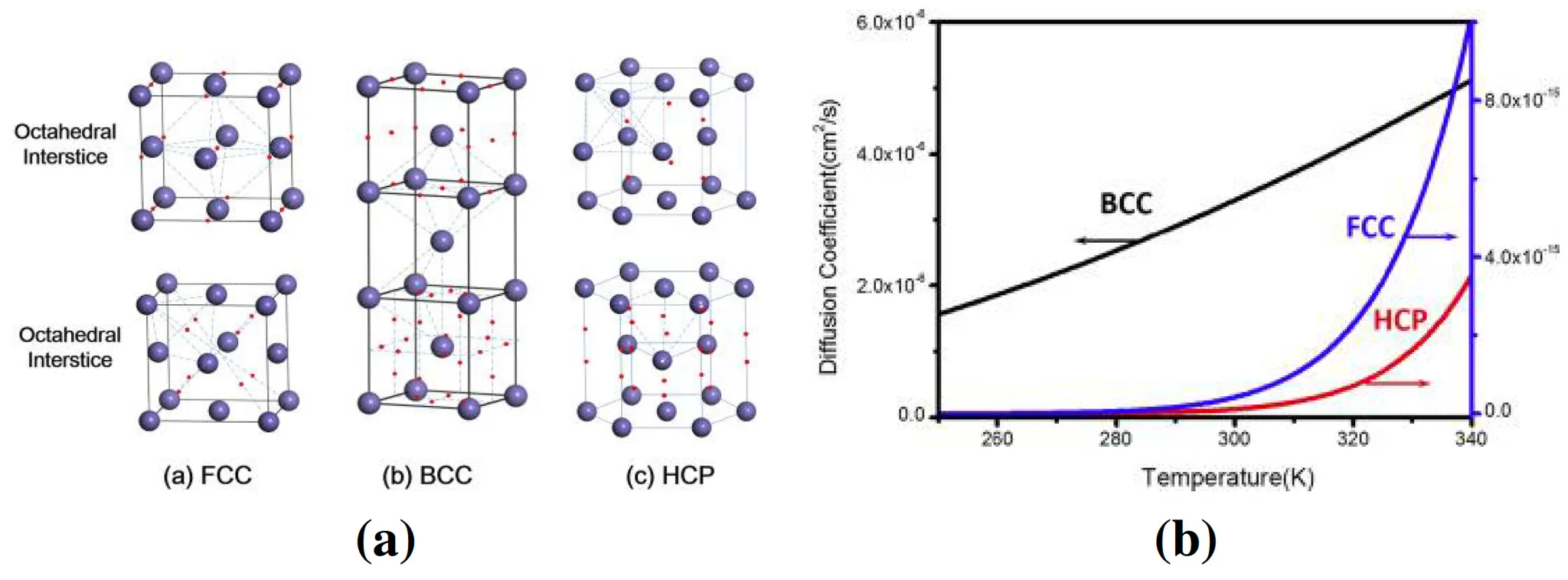
The crystal structure of metals (BCC, FCC, HCP) influences the pathways and speed of hydrogen diffusion:
•
FCC Structures (e.g., Hastelloy X): Hydrogen diffusion is relatively complex, but the structural stability makes it well-suited for high-temperature and high-pressure environments.
•
BCC Structures (some Aluminum alloys): Hydrogen diffusion is faster, but the high diffusion rate can increase susceptibility to embrittlement.
Figure 5: ( Location of O octahedral site (O site) and Tetrahedral site (T site) in Face Centred Cubic (FCC), Body Centred Cubic (BCC) and Hexagonal Close Packed (HCP) [9], (right) Temperature and diffusion
coefficient graph of theoretical calculation by He et al using Arrhenius diffusion equation
2.
Role of Trapping Sites
Hydrogen becomes trapped at specific locations within the metal (e.g., grain boundaries, precipitates), significantly impacting the metal’s physical and chemical properties:
•
Al6082-T6: Precipitates such as and , formed during heat treatment, serve as primary trapping sites, potentially increasing hydrogen trapping and inducing cracks and embrittlement.
•
Hastelloy X: Cr-rich carbides reinforce grain boundaries and inhibit hydrogen diffusion, providing a protective role.
This theoretical framework provides the basis for analyzing how hydrogen diffusion and trapping mechanisms affect the durability and performance of the two alloys.
Logical Progression of the Study
Based on the theoretical framework, this study followed the following logical steps to design experiments and achieve its objectives:
1.
Analysis of Hydrogen Absorption and Trapping Mechanisms
•
Expose Al6082-T6 and Hastelloy X to high-pressure (18 bar) and high-temperature (473K, 603K) hydrogen environments.
•
Observe microstructural changes and analyze the extent to which precipitates and carbides act as hydrogen trapping sites.
2.
Comparison of Hydrogen Diffusion Characteristics
•
Use Intelligent Gravimetric Analysis (IGA) to quantitatively measure hydrogen absorption rates and weight changes in the two alloys.
•
Conduct XRD analysis to determine the impact of hydrogen diffusion on the crystal structure of each metal.
3.
Durability Evaluation
•
Evaluate the physical and chemical stability of both alloys under high-temperature and high-pressure hydrogen conditions.
•
While Hastelloy X demonstrates stability through Cr-rich carbides that suppress hydrogen diffusion, Al6082-T6 shows potential susceptibility to cracking due to trapping effects.
4.
Assessment of Applicability in Hydrogen Fuel Systems
•
Based on experimental data, assess the suitability of the two alloys as materials for hydrogen fuel delivery systems in high-pressure and high-temperature environments.
Literature Review and Limitations
Literature Review
1.
Al6082-T6
•
Previous studies have shown that Al6082-T6 is advantageous in terms of lightweight and cost efficiency. However, under high-pressure and high-temperature hydrogen environments, precipitates such as and act as hydrogen trapping sites, increasing the likelihood of cracking and structural instability.
2.
Hastelloy X
•
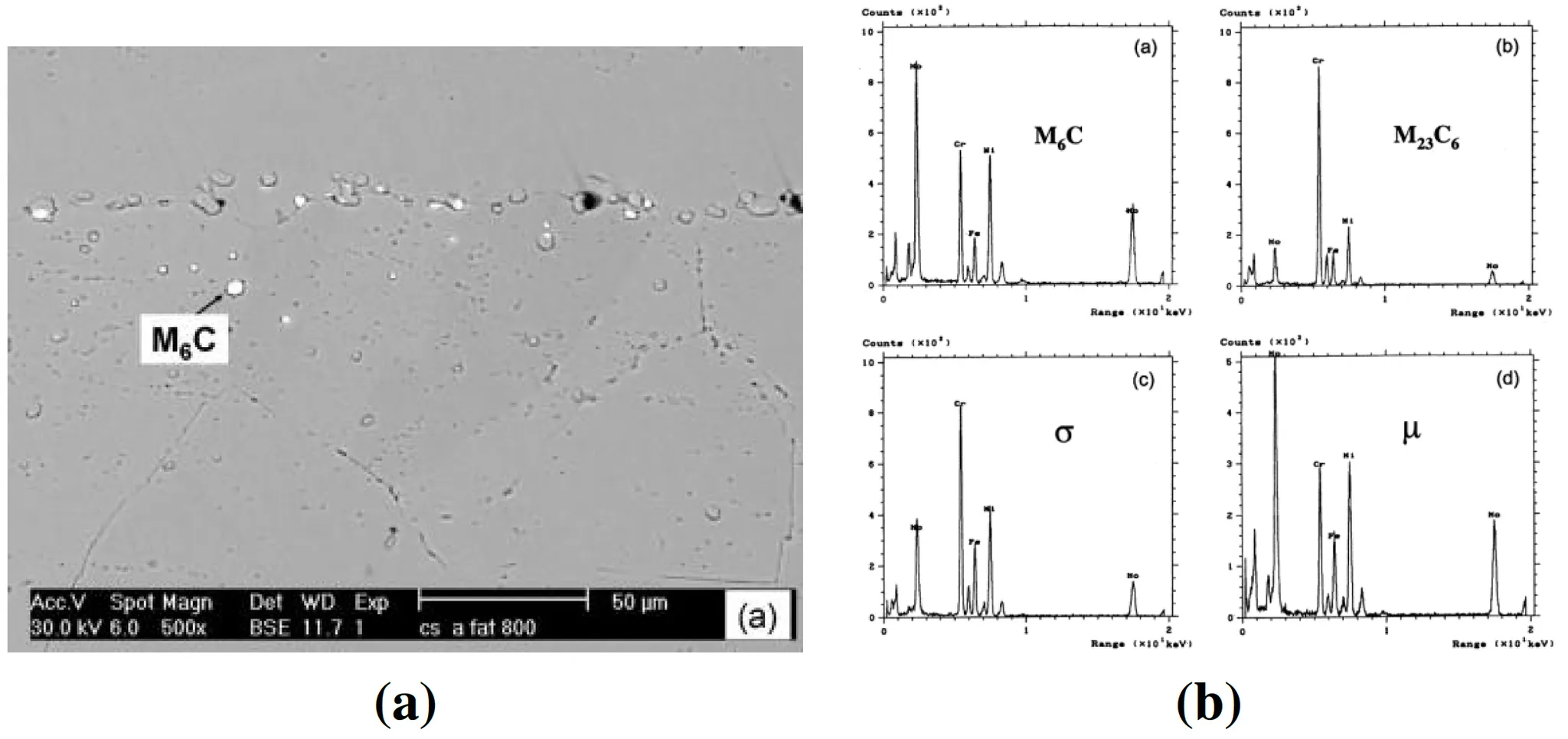
As a nickel-based superalloy, Hastelloy X is predominantly used in high-temperature applications. Cr-rich carbides formed at grain boundaries play a crucial role in suppressing hydrogen diffusion, enhancing its durability under extreme conditions.
•
Existing studies confirm Hastelloy X’s high strength and stability in high-pressure and high-temperature environments, making it suitable for aircraft turbine engines.
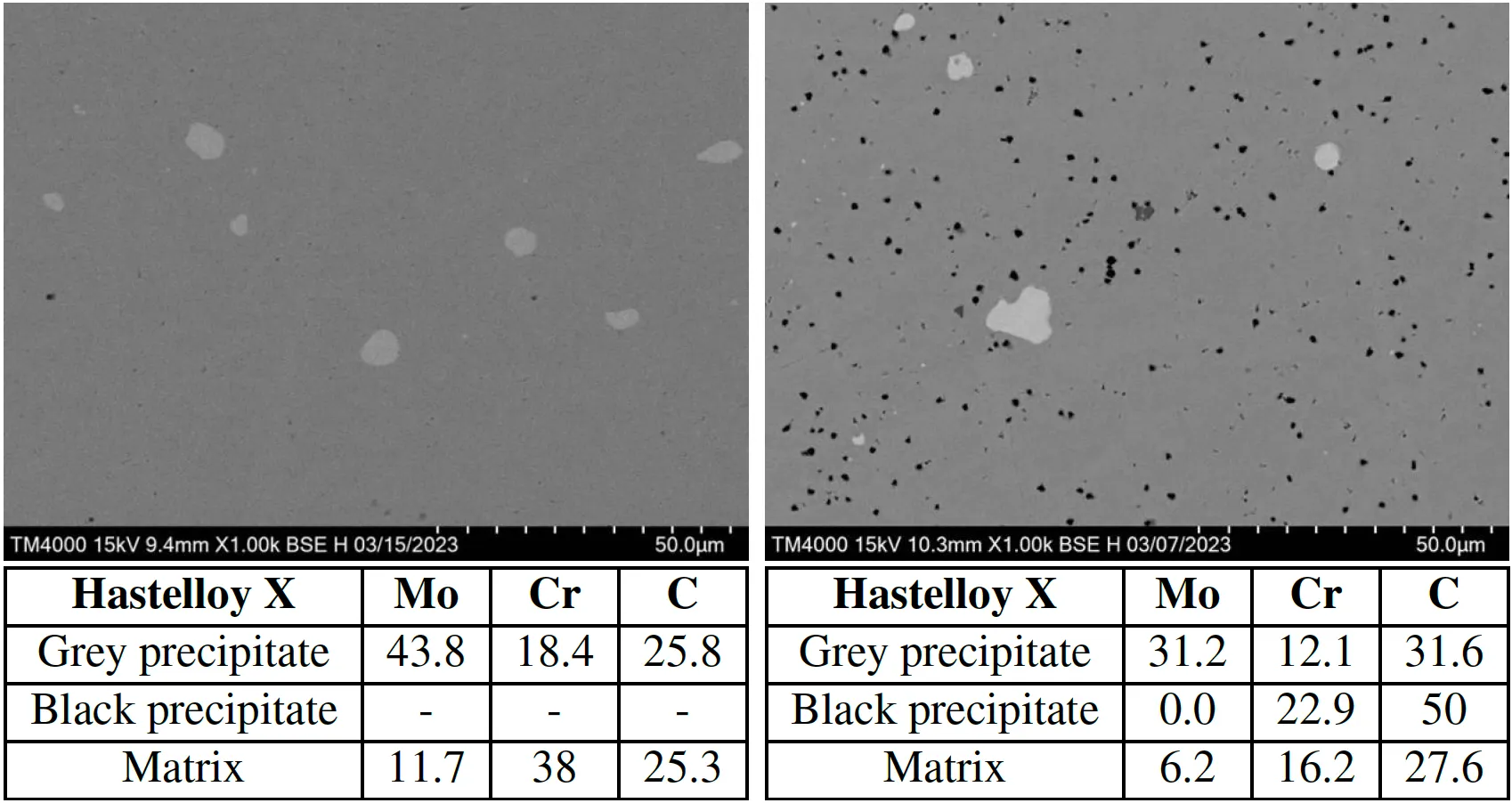
Figure 6: (left) SEM microstructure image of Hastelloy X with precipitation (white), (right) EDX analysis of each precipitation forming in Hastelloy X: (a) , (b) , (c) σ phase, and (d) μ phase
Limitations
1.
Sample Size and Experimental Conditions
•
The study was conducted under specific temperature (473K, 603K) and pressure (18 bar) conditions, limiting the generalization of results to more diverse operational environments.
2.
Lack of Long-Term Exposure Studies
•
The short duration of the study limits the ability to evaluate the long-term physical and chemical changes caused by exposure to hydrogen environments.
•
Examples include metal fatigue and changes due to repeated hydrogen charging/discharging cycles.
3.
Insufficient Comparative Research
•
While prior studies have focused on Al6082-T6 and Hastelloy X individually, few studies have directly compared these two alloys under identical conditions. This research addresses this gap by conducting a direct performance comparison in hydrogen environments.
Differences
This study distinguishes itself from previous research through the following key differences:
1.
Direct Comparative Experimental Design
•
By testing Al6082-T6 and Hastelloy X under identical high-pressure and high-temperature conditions, the study directly compares their microstructural changes, hydrogen trapping mechanisms, and durability.
2.
Quantitative Hydrogen Absorption and Diffusion Data
•
The use of Intelligent Gravimetric Analysis (IGA) provided precise quantitative data on hydrogen absorption rates and weight changes for both alloys.
3.
Evaluation of Applicability in Aviation
•
This research evaluates the potential of both alloys for hydrogen-based aviation fuel systems, offering practical insights into material selection for high-pressure and high-temperature hydrogen environments.
Methodology
Research Design
Data Collection Methods
1.
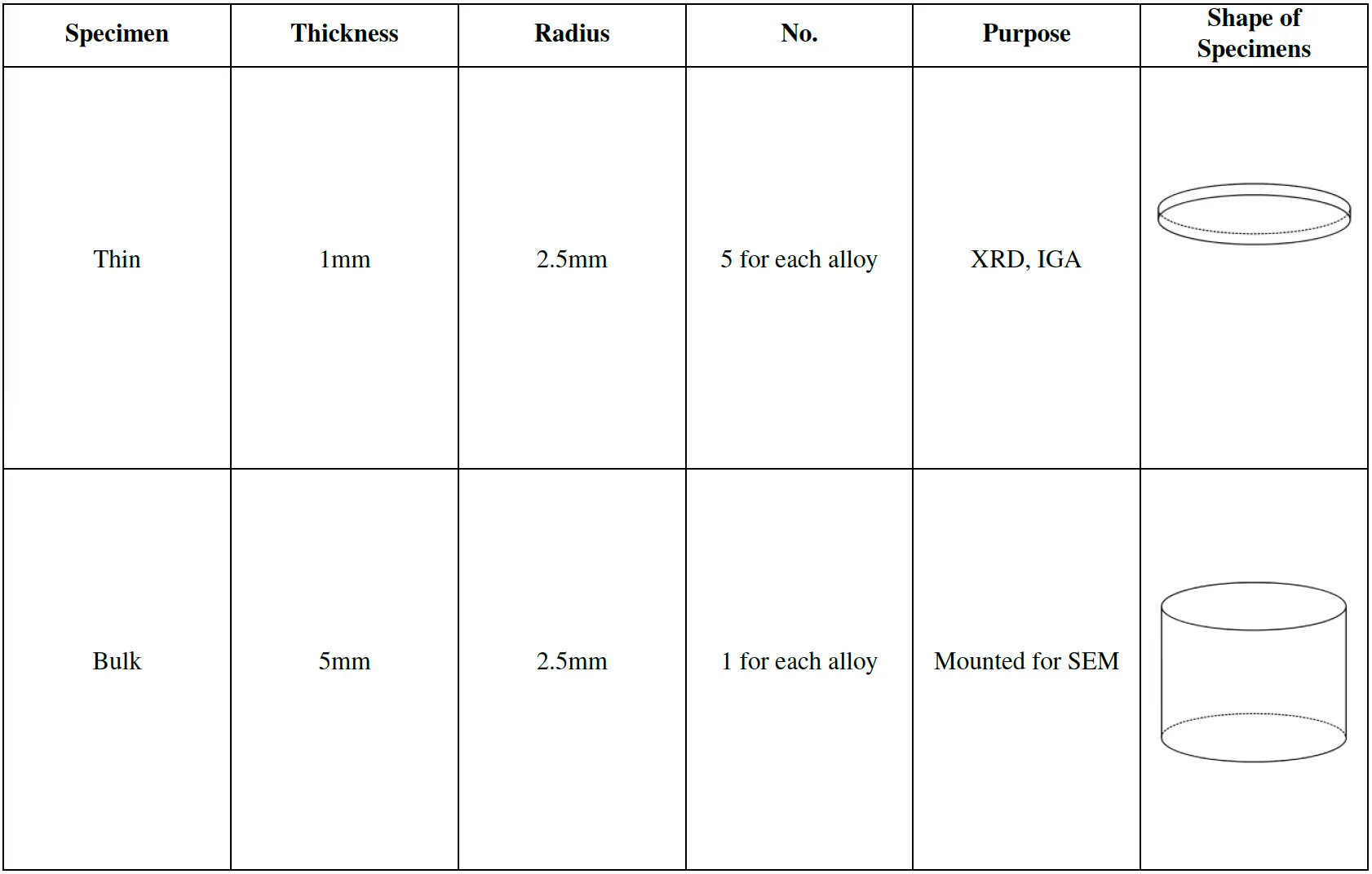
Sample Preparation
•
Materials: Aluminium alloy 6082-T6 and Nickel-based Hastelloy X were prepared as specimens.
•
Shapes: Thin (1mm) and bulk (5mm) samples were prepared for different tests, including XRD, SEM, and IGA.
•
Grinding and Polishing:
◦
Pre-H-exposure: Polished using MD-Dac (diamond suspension) and MD-Chem (oxide suspension).
◦
Post-H-exposure: Adjusted procedures were followed for Al6082-T6 and Hastelloy X.
Table 5: Sample information used for the experiment and their purposes
2.
Microstructural Observation
•
Scanning Electron Microscopy (SEM): Used for analyzing surface microstructures.
•
Energy Dispersive X-ray (EDX): Examined chemical compositions of precipitates.
•
X-ray Diffraction (XRD): Measured lattice distortions and crystalline structural changes using Cu-Kα radiation.
3.
Hydrogen Absorption
•
Intelligent Gravimetric Analysis (IGA):
◦
Conducted under high-pressure hydrogen (18 bar) and two elevated temperatures (473 K and 603 K).
◦
Monitored mass changes due to hydrogen adsorption.
◦
Recorded weight, pressure, and temperature over 1000–6000 hours.
Data Analysis Procedures
1.
Microstructural Analysis
•
SEM images were processed with Image J to quantify precipitate size changes and coarsening.
•
EDX analysis provided compositional data for precipitates (e.g., in Al6082-T6 and Cr-rich in Hastelloy X).
2.
Crystalline Structure Investigation
•
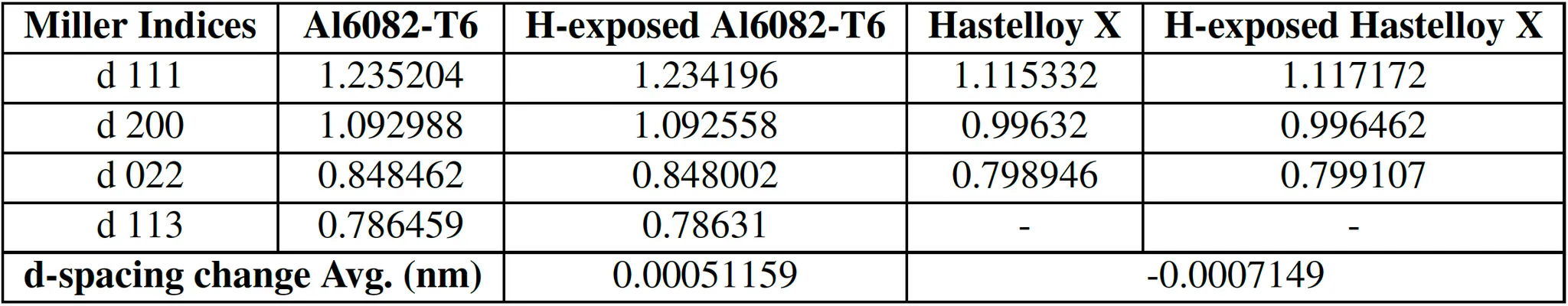
Bragg's Law: Calculated lattice parameters and d-spacing values before and after hydrogen exposure.
•
Peak Broadening: Assessed through Williamson-Hall plots to identify crystallite size and microstrain variations.
3.
Gravimetric Analysis
•
The temperature dependence of hydrogen absorption was quantified through weight changes in the IGA system.
•
Trends between pressure and weight were linearly analyzed.
4.
Comparative Observations
•
Compared H-exposed vs. non-H-exposed microstructures and XRD patterns for Al6082-T6 and Hastelloy X.
•
Differences in void formation (Al6082-T6) and carbide precipitation behavior (Hastelloy X) were evaluated.
Result
Key Results
1.
Aluminium Alloy 6082-T6:
•
Microstructure:
◦
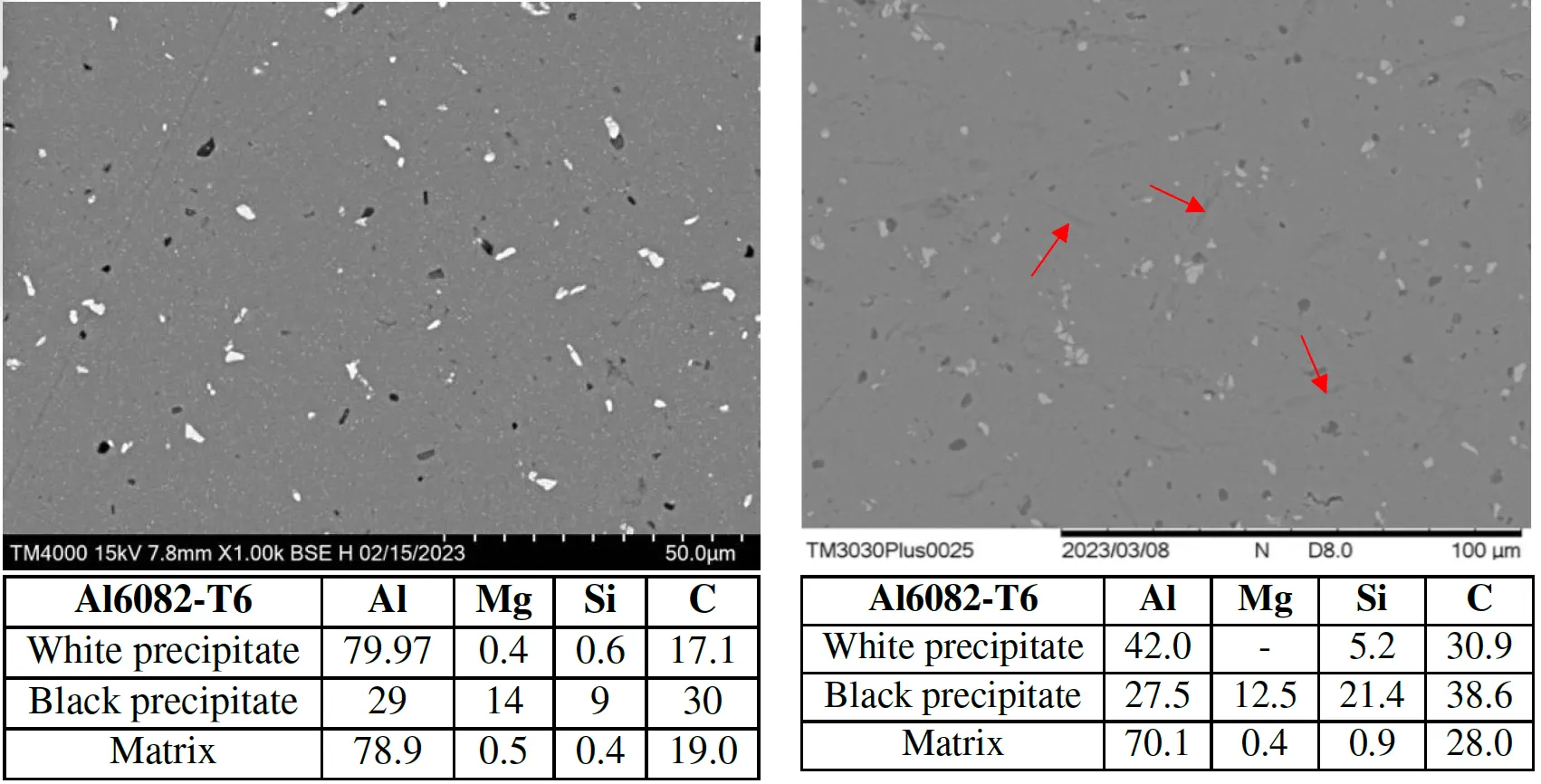
Precipitate coarsening observed in Al(MnFe)Si and Al-Mg2Si phases.
◦
Formation of longitudinal lattice distortion and voids near precipitates was identified, indicating a susceptibility to hydrogen-induced cracking.
•
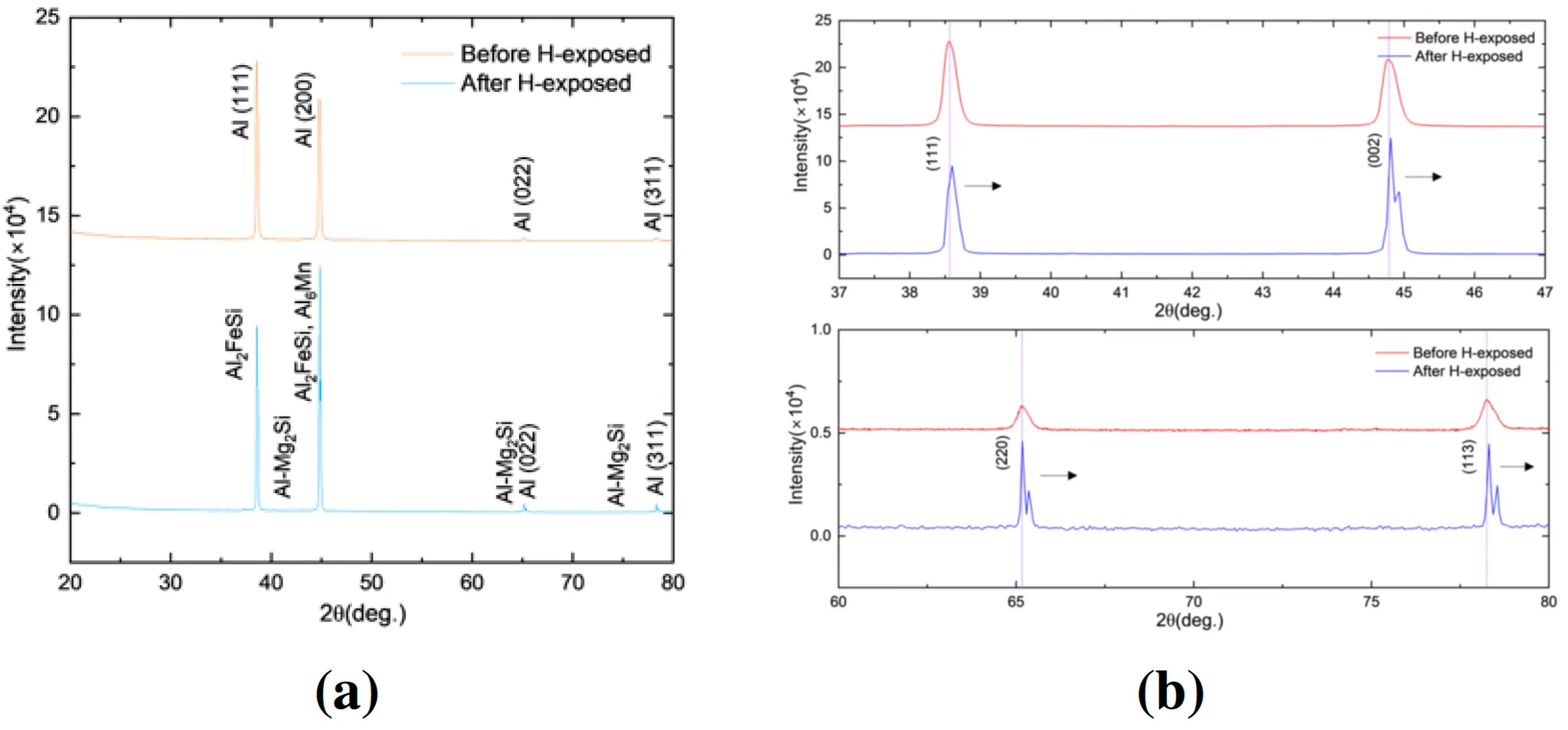
XRD Analysis:
◦
Significant peak broadening and lattice contraction were detected after hydrogen exposure. These suggest that hydrostatic pressure and temperature influenced the crystalline structure.
•
Gravimetric Analysis:
◦
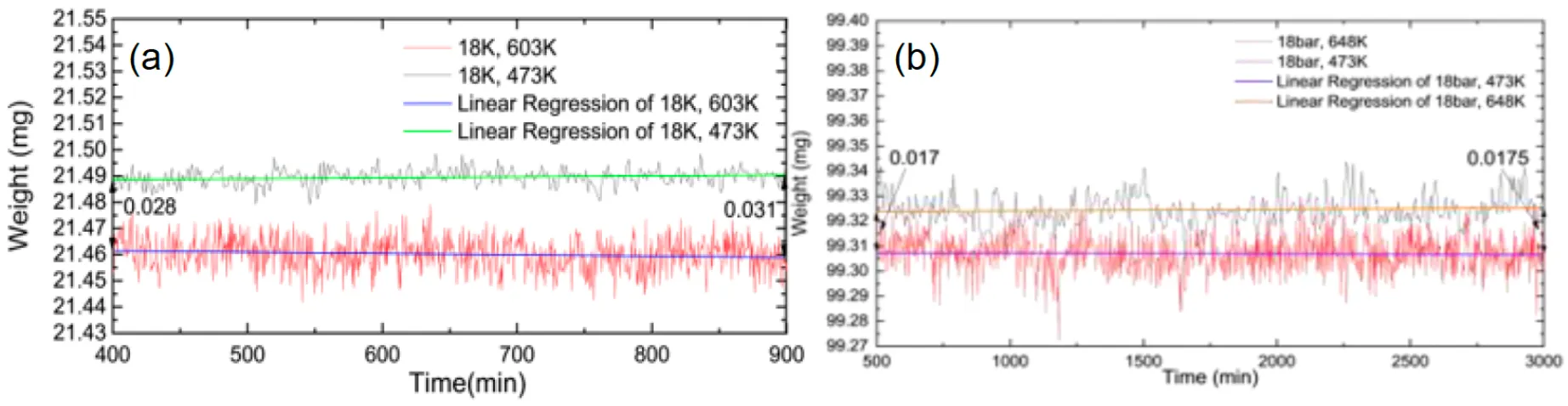
Weight reduction of 0.12% at 473 K and 0.25% at 603 K, showing a strong temperature dependency for hydrogen absorption.
◦
Higher sensitivity to hydrogen exposure compared to Hastelloy X.
Figure 9: Scanning Electron Microstructure images of Al6082-T6 microstructure of before H-exposure (left) and after the H-exposure at 18bar, 473K (Red arrows: indicate longitudinal voids), and EDX analysis.
Figure 10: (left) XRD pattern comparison before H exposure and after H exposed of Al6082 T6 from 20◦ to 80◦ with the peak identity , and (separated XRD pattern of Al6082 T6 from 37◦ to 47◦ and 60◦ to 80◦ (Purple line: indicating line for specific peak
2.
Hastelloy X:
•
Microstructure:
◦
Formation of Cr-rich M23C6 carbide along grain boundaries was identified after hydrogen exposure.
◦
No visible voids, blisters, or lattice distortion, indicating strong resistance to hydrogen embrittlement.
•
XRD Analysis:
◦
No significant peak shifting or lattice distortion observed, even after exposure to elevated pressure and temperature.
•
Gravimetric Analysis:
◦
Weight reduction of 0.07% for a temperature increase of 150 K, showcasing lower hydrogen uptake compared to Aluminium 6082.
Figure 12: Scanning Electron Microstructure images of Hastelloy X microstructure of before H-exposure (left) and after the H-exposure (right) at 18bar, 473K, and EDX analysis.
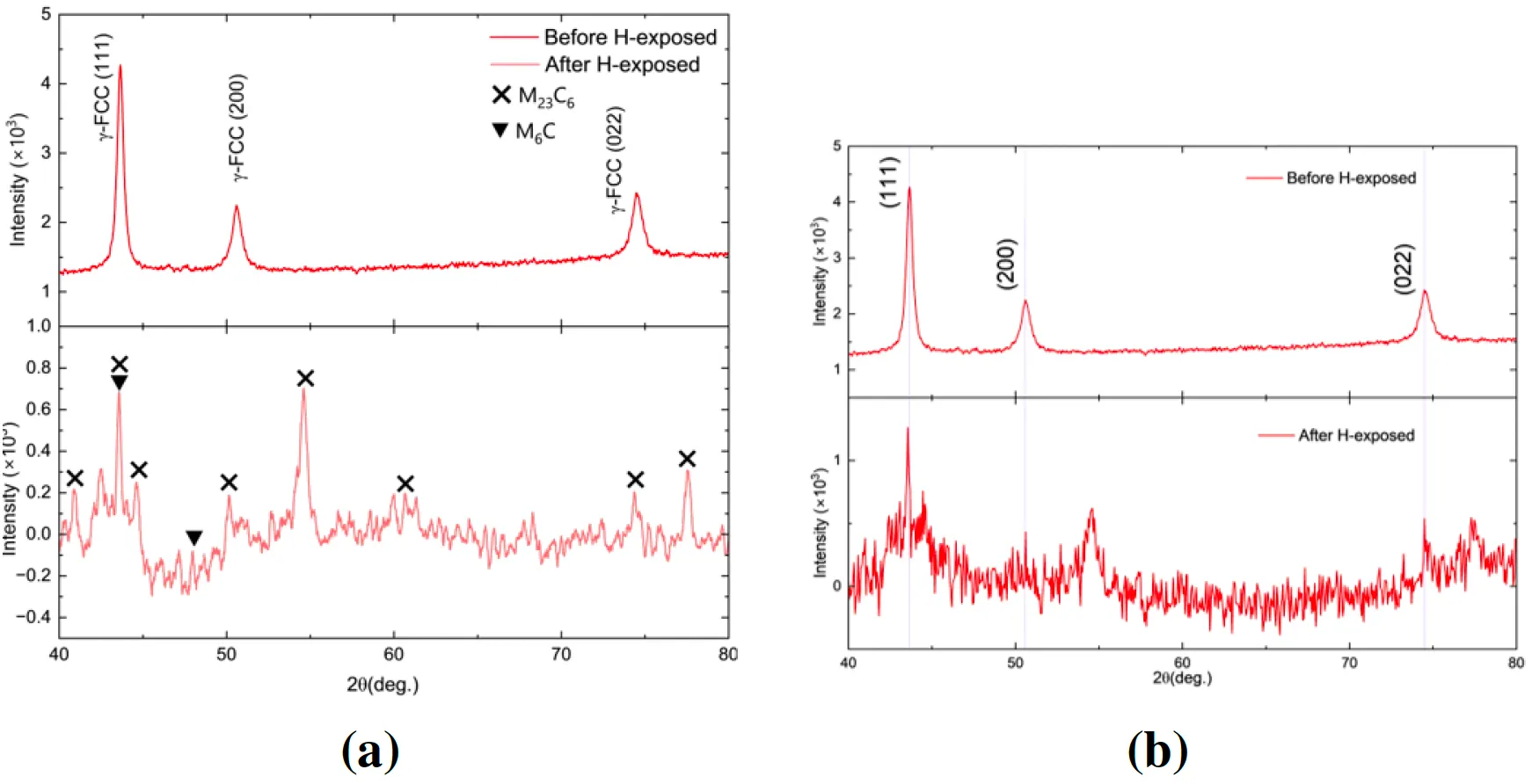
Figure 14: (left) XRD pattern peak identification using previous research
and programme MATCH!, and (right) XRD pattern comparison between
H exposed Hastelloy X at 18bar, 473K and non H exposed Hastelloy X
with the FCC peaks.
Discussion
Interpretation of Results
Hydrogen-Induced Microstructural Changes
1.
Aluminium Alloy 6082-T6:
•
Precipitate Behavior: Hydrogen exposure led to the coarsening of Al-Mg2Si and Al(MnFe)Si precipitates, which act as hydrogen trapping sites. These sites promote localized hydrogen accumulation, increasing the risk of microcracks.
•
Void Formation: Significant voids were observed near precipitates after hydrogen exposure, particularly at higher temperatures. This indicates that hydrogen embrittlement in Al6082-T6 is strongly dependent on temperature, making it less suitable for high-temperature hydrogen environments.
2.
Hastelloy X:
•
Carbide Formation: The Cr-rich M23C6 carbide formation along grain boundaries enhanced the alloy’s resistance to hydrogen diffusion. This effectively inhibited void formation and maintained microstructural stability, even under high-temperature, high-pressure hydrogen conditions.
•
Grain Boundary Strength: The stabilization provided by M23C6 carbides suggests that Hastelloy X can reliably prevent hydrogen-induced degradation, making it a preferred material for extreme conditions.
Crystalline Structure and Hydrogen Diffusion
1.
XRD Findings:
•
Al6082-T6 exhibited significant peak broadening and lattice contraction after hydrogen exposure, particularly at 603 K. This suggests notable hydrogen-induced strain within the lattice, reducing its mechanical stability under these conditions.
•
In contrast, Hastelloy X showed minimal changes in its XRD patterns, indicating that its lattice structure remains largely unaffected by hydrogen exposure. This aligns with its high resistance to hydrogen embrittlement.
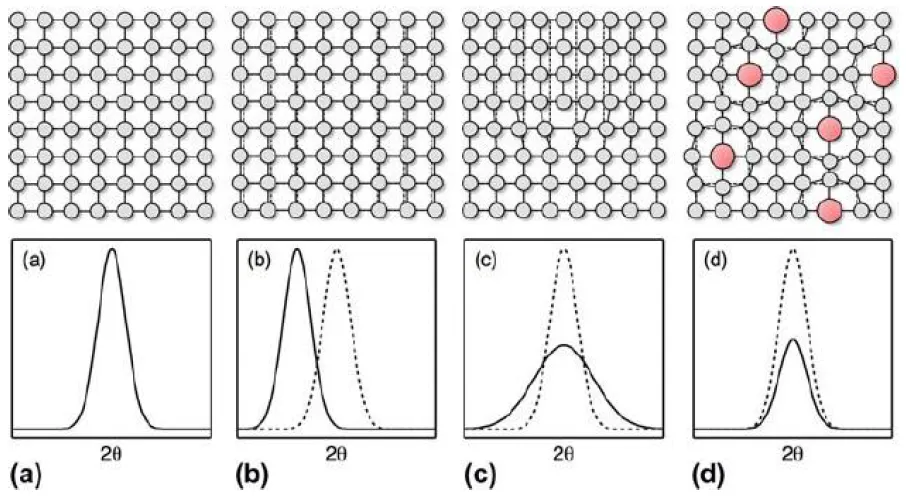
Figure 24: Bragg peaks corresponding lattice distortion situations in single crystal (a) Perfect crystal, (b) peak shifting due to interplanar contraction or expansion, (c) peak broadening due to interplanar spacing by planar defect, and (d) peak size reduction due to point defect
Table 7: d-spacing calculation result of Al6082-T6 and Hastelloy X FCC indices using Bragg’s law
2.
Hydrogen Diffusion Behavior:
•
Temperature Sensitivity: Hydrogen diffusion in Al6082-T6 increased significantly with temperature, as evidenced by a greater weight reduction (up to 0.25% at 603 K). This highlights its vulnerability in high-temperature hydrogen environments.
•
Hastelloy X exhibited a much lower hydrogen absorption rate, with only a 0.07% weight reduction, confirming its superior performance in resisting hydrogen diffusion.
Figure 27: IGA Result and linear regression of each result that shows trends of weight changes at different times: (a) Al6082 T6, (b) Hastelloy X
Material Suitability for Hydrogen Applications
1.
Al6082-T6:
•
While Al6082-T6 is cost-effective and lightweight, its high susceptibility to hydrogen-induced microstructural changes limits its use in high-pressure hydrogen systems. However, it may still be applicable in low-pressure hydrogen environments where temperature variations are minimal.
2.
Hastelloy X:
•
The superior performance of Hastelloy X in resisting hydrogen-induced degradation makes it a highly suitable candidate for high-temperature and high-pressure hydrogen systems, such as those near fuel injectors in hydrogen-powered aviation.
Study Limitations
1.
Experimental Scope:
•
The study focused solely on the gaseous hydrogen phase. The potential effects of liquid or solid hydrogen on microstructure and diffusion behavior were not addressed.
•
The limited range of pressure (18 bar) and temperature (473 K to 603 K) restricts the application of findings to environments beyond these conditions.
2.
Short Exposure Duration:
•
Only short-term hydrogen exposure was evaluated, and cumulative effects such as hydrogen fatigue or long-term degradation were not analyzed.
3.
Data Interpretation Constraints:
•
The study's observations primarily focused on microstructural and gravimetric changes. Additional quantitative metrics, such as diffusion coefficients or activation energies, would provide deeper insights.
Suggestions for Future Research
Recommendations for Future Research
1.
Extended Testing Parameters:
•
Conduct long-term hydrogen exposure experiments to assess cumulative effects on both alloys, including microstructural evolution over time.
•
Expand the range of experimental conditions to include extreme temperatures and pressures representative of real-world hydrogen applications.
2.
Process Optimization:
•
Investigate advanced surface treatments or protective coatings for Al6082-T6 to reduce hydrogen absorption and mitigate embrittlement risks.
•
Explore heat treatment modifications for Hastelloy X to further enhance its grain boundary stability and resistance to hydrogen diffusion.
3.
Hydrogen Diffusion Analysis:
•
Perform in-depth diffusion studies to determine hydrogen diffusion coefficients and activation energies for both alloys, providing a clearer understanding of their behavior under varying conditions.
4.
Cross-Phase Studies:
•
Explore the effects of liquid and solid hydrogen phases on the microstructure and mechanical properties of these alloys to validate their performance in diverse hydrogen storage and delivery systems.
Conclusion
Research Findings
This thesis investigated the behavior of two aerospace alloys, Al6082-T6 and Hastelloy X, in high-purity hydrogen environments under elevated temperature and pressure (18 bar, 473K-648K). Key findings include:
•
Al6082-T6: Exhibited significant lattice distortion and precipitate coarsening, indicating potential susceptibility to hydrogen embrittlement. Gravimetric analysis demonstrated a temperature-dependent weight reduction, highlighting its limited performance in higher-pressure hydrogen environments.
•
Hastelloy X: Showed the formation of Cr-rich M23C6 carbides without lattice distortion or signs of hydrogen embrittlement. It demonstrated greater stability under similar conditions, with less weight reduction and better resistance to hydrogen-induced effects.
Research Contribution
This study contributed to:
1.
Establishing microstructural and crystallographic differences between Al6082-T6 and Hastelloy X when exposed to hydrogen.
2.
Proposing Hastelloy X as a more suitable material for high-pressure hydrogen delivery systems, while highlighting the limitations of Al6082-T6 for such applications.
3.
Providing a foundation for the application of hydrogen in aerospace fuel delivery systems as a replacement for kerosene, emphasizing material performance under real-world conditions.
Final Remarks
The transition to hydrogen energy represents a transformative step toward sustainability in the aerospace industry, offering a cleaner and more efficient alternative to kerosene-based fuels. This research highlights the critical importance of material selection in ensuring the safety and efficiency of hydrogen delivery systems, with Hastelloy X emerging as a highly promising candidate due to its superior resistance to hydrogen-induced degradation. The findings underscore the pivotal role of materials science in enabling hydrogen-powered aviation, paving the way for a greener and more sustainable future. However, further research is essential to address challenges such as long-term durability, varied environmental conditions, and the scalability of hydrogen-based systems to realize the full potential of this revolutionary technology.
Reference
Appendix
🩻 XRD Analysis
Advancing the transition of commercial aviation to a low-carbon future requires investments in promising technologies, including alternate forms of propulsion. - Robert Isom, CEO of American Airlines -